Quality Manufacturer In Bulk Supply Wholesale Vardenafil 224785-90-4
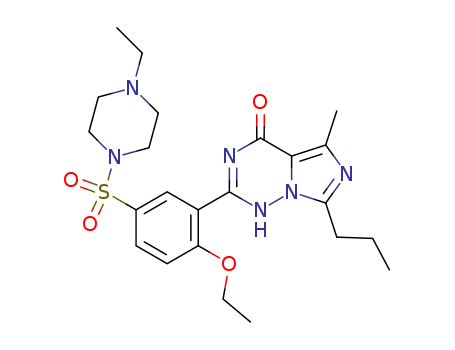
- Molecular Formula:C23H32N6O4S
- Molecular Weight:488.611
- Appearance/Colour:White crystalline powder
- Vapor Pressure:5.17E-19mmHg at 25°C
- Melting Point:230-235 °C
- Boiling Point:692.2 °C at 760 mmHg
- PKA:9.86±0.20(Predicted)
- Flash Point:372.5 °C
- PSA:121.28000
- Density:1.37 g/cm3
- LogP:4.63100
Vardenafil hydrochloride trihydrate(Cas 224785-90-4) Usage
|
Originator
|
Bayer AG (Germany)
|
|
Uses
|
Vardenafil is a PDE5 inhibitor used for treating male erectile dysfunction. It shares structural similarities with sildenafil (Viagra), the first PDE5 inhibitor introduced for this purpose in 1998. Vardenafil is synthesized in three steps, with an initial cyclization reaction followed by sulfonation and condensation steps. It exhibits potent PDE5 inhibition, with a significantly lower IC50 (0.7 nM) compared to sildenafil (6.6 nM). Vardenafil is typically administered in 10 or 20 mg doses, with a short time to reach peak plasma concentration (0.75 h) and a 4–5-hour half-life. It undergoes extensive first-pass metabolism in the liver, primarily by CYP3A4, and is primarily eliminated in feces. Clinical studies have shown it to be well-tolerated and effective, even in patients with comorbidities like diabetes or hypertension. Common side effects include headache, flushing, dyspepsia, and nasal congestion. Although vardenafil has vasodilatory properties, it generally does not lead to clinically significant blood pressure changes. It has the potential for interactions with certain drugs, and its metabolism primarily involves CYP3A4. The primary metabolite of vardenafil also exhibits some activity, and most of the drug is excreted in feces. |
|
Definition
|
ChEBI: The sulfonamide resulting from formal condensation of the sulfo group of 4-ethoxy-3-(5-methyl-7-propylimidazo[5,1-f][1,2,4]triazin-4(1H)-one-2-yl)benzenesulfonic acid and the secondary amino group of 4-ethylpiperazine.
|
|
Brand name
|
Levitra
|
|
Flammability and Explosibility
|
Nonflammable
|
|
Clinical Use
|
Treatment of erectile dysfunction
|
InChI:InChI=1/C23H32N6O4S.ClH.3H2O/c1-5-8-20-24-16(4)21-23(30)25-22(26-29(20)21)18-15-17(9-10-19(18)33-7-3)34(31,32)28-13-11-27(6-2)12-14-28;;;;/h9-10,15H,5-8,11-14H2,1-4H3,(H,25,26,30);1H;3*1H2
224785-90-4 Relevant articles
A METHOD FOR THE PREPARATION AND ISOLATION OF SALTS OF VARDENAFIL WITH ACIDS
-
Page/Page column 17, (2013/06/06)
The subject of this invention provides a...
Vardenafil, a New Phosphodiesterase Type 5 Inhibitor, in the Treatment of Erectile Dysfunction in Men With Diabetes: A multicenter double-blind placebo-controlled fixed-dose study
Irwin Goldstein, MD; Jay M. Young, MD; Jerome Fischer, MD; Keith Bangerter, PHD; Thomas Segerson, MD; Terry Taylor, MD;
, Diabetes Care 2003;26(3):777–783
Both vardenafil doses significantly enhanced the rates of successful penetration (P < 0.0001) and successful intercourse (P < 0.0001) compared with placebo. Vardenafil treatment was effective in increasing intercourse success rates at all levels of baseline ED severity, at each level of plasma HbA1c, and for type 1 and 2 diabetes. Treatment-emergent adverse events were primarily mild to moderate headache (≤13%), flushing (≤10%), and rhinitis (≤10%).
The phosphodiesterase-5 inhibitor vardenafil reduces oxidative stress while reversing pulmonary arterial hypertension
You-Fei Fan, Rui Zhang, Xin Jiang, Li Wen, Dan-Chen Wu, Dong Liu, Ping Yuan, Yu-Lin Wang, Zhi-Cheng Jing
, Cardiovascular Research, Volume 99, Issue 3, 1 August 2013, Pages 395–403
In a study involving both rats with monocrotaline-induced pulmonary arterial hypertension (PAH) and PAH patients, the effects of vardenafil were investigated. Rats were administered oral vardenafil, while patients received vardenafil orally for a specified duration. The results showed that vardenafil led to significant improvements in both groups. It reduced pulmonary vascular resistance, increased cardiac output, and had a positive impact on hemodynamic parameters.
224785-90-4 Process route
-
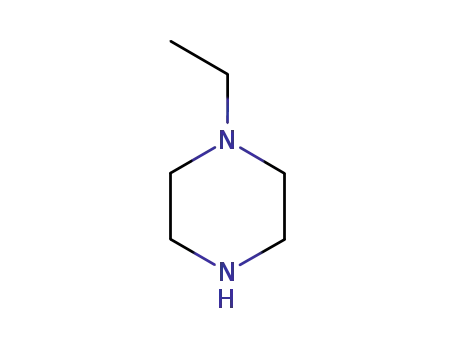
- 5308-25-8
4-ethylpiperazine
-
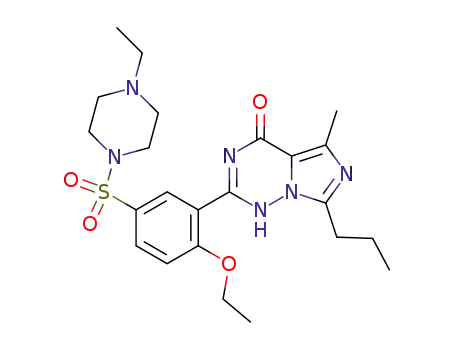
- 224789-21-3
2-(2-ethoxy-phenyl)-5-methyl-7-propyl-3H-imidazo[5,1-f][1,2,4]triazin-4-one
Conditions
| Conditions |
Yield |
|
2-(2-ethoxy-phenyl)-5-methyl-7-propyl-3H-imidazo[5,1-f][1,2,4]triazin-4-one; With chlorosulfonic acid; at 0 - 22 ℃; for 0.75h;
4-ethylpiperazine; In dichloromethane; at -3 - 25 ℃; for 0.75h; Product distribution / selectivity;
|
|
-
- 5308-25-8
4-ethylpiperazine
-
- 224789-26-8
4-ethoxy-3-(5-methyl-4-oxo-7-propyl-3,4-dihydroimidazo[5,1-f][1,2,4]triazin-2-yl)-benzenelsulfonic acid chloride
Conditions
| Conditions |
Yield |
|
In dichloromethane;
|
|


![2-(2-ethoxy-phenyl)-5-methyl-7-propyl-3H-imidazo[5,1-f][1,2,4]triazin-4-one](/upload/2023/8/9a4d56e8-ab30-4988-a1b6-23fa0421f7a9.png)

![4-ethoxy-3-(5-methyl-4-oxo-7-propyl-3,4-dihydroimidazo[5,1-f][1,2,4]triazin-2-yl)-benzenelsulfonic acid chloride](/upload/2023/8/100666fc-1d36-45f9-ad73-519b84ddfe63.png)