|
Chemical Properties
|
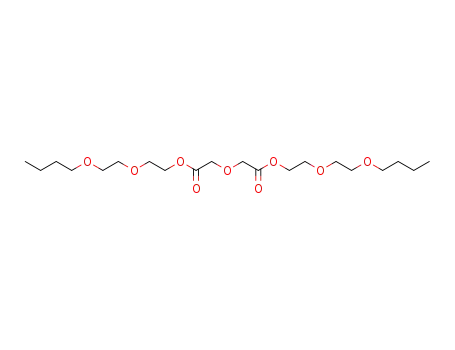
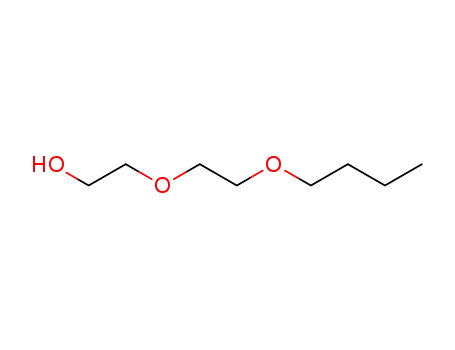
Diethylene glycol monobutyl ether is a colorless, high-boiling liquid with a mild odour. It is miscible in proportions with water, alcohol (methanol), ketones (acetone), ethers (ethyl ether), aromatic hydrocarbons (benzene), paraffinic hydrocarbons (n-heptane), and halogenated hydrocarbons (carbon tetrachloride). As it is an ether-alcohol type compound it possesses solvent action for many substances such as oils, dyes, gums, and natural and synthetic resins. It is used as a high-boiling solvent in nitrocellulose lacquers and other synthetic coatings, baking lacquers, flash-dry printing inks, and dye bath. |
|
Uses
|
Diethylene glycol monobutyl ether(DGBE) is widely used as a solvent for cellulose ester, lacquers, varnishes, cleaners, detergents, dyes, ink, and paint industries. it is also used as an intermediate for plasticizers and a diluent for hydraulic brake fluids, in addition to the production of piperonyl butoxy compounds. In France, its use in the cosmetics industry is permissible, wherein it is used as a solvent in hair dyes with a maximum concentration of 9%. |
|
Application
|
Diethylene glycol mono-n-butyl ether has a wide variety of applications in Chiral chemistry and green chemistry. It is also used in cosmetics. It is used as diluents and leveling agents in the manufacture of paints and in baking. It is also used in the manufacture of nitrocellulose. In brake fluid, it is used as an additive. It is used in the printing industry due to its slow evaporation rate. It is also used as a fixative for perfumes and antiseptics. It is used as an additive to prevent ice buildup in jet fuel. |
|
Preparation
|
Diethylene glycol monobutyl ether is prepared by co-heating ethylene oxide and ethylene glycol butyl ether under pressure. |
|
General Description
|
Diethylene glycol monobutyl ether is a colorless liquid with a mild pleasant odor. Mixes with water. (USCG, 1999) |
|
Air & Water Reactions
|
Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick, 1979 p.151-154, 164]. Water soluble. |
|
Reactivity Profile
|
Butyldiglycol is a ether-alcohol derivative. The ether being relatively unreactive. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert alcohols to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides. |
|
Health Hazard
|
Diethylene glycol monobutyl ether(DGBE) is an eye irritant. It showed low toxicity in test species.Toxic symptoms are similar to those ofother glycol ethers containing two etherealoxygen atoms. Inhalation for brief periods has no significant effect. Contact with liquid causes moderate irritation of eyes and corneal injury. Prolonged contact with skin causes only minor irritation. The high dosescaused pulmonary congestion. No renaldamage was reported. There is no reporton teratogenicity of this compound. |
|
Fire Hazard
|
Butyldiglycol is combustible. |
|
Flammability and Explosibility
|
Nonflammable |
|
Contact allergens
|
This organic solvent belongs to the carbitols group and
is included in waterbased liquids such as paints, surface
cleaners, polishes, and disinfectants. It is considered
to be an exceptional allergen. |
|
Safety Profile
|
Moderately toxic by
ingestion and intraperitoneal routes. Mddly
toxic by skin contact. A severe eye irritant.
Combustible when exposed to heat or
flame; can react with oxidizing materials. To
fight fire, use alcohol foam, CO2, or dry
chemical. When heated to decomposition it
emits acrid smoke and irritating fumes. See
also GLYCOL ETHERS. |
|
Purification Methods
|
Dry the ether with anhydrous K2CO3 or CaSO4, filter and fractionally distil it. Peroxides can be removed by refluxing with stannous chloride or a mixture of FeSO4 and KHSO4 (or, less completely, by filtration under slight pressure through a column of activated alumina). [Beilstein 1 IV 2394.] |
|
Waste Disposal
|
DGBE is mixed with a combustible solventand burned in a chemical incinerator. Smallamounts may be disposed down the drainwith large amounts of water. |
|
Consumer Uses
|
This substance is used in the following products:
coating products,
washing & cleaning products,
fertilisers,
heat transfer fluids,
hydraulic fluids,
cosmetics and personal care products,
lubricants and greases,
plant protection products,
water treatment chemicals and perfumes and fragrances. Other release to the environment of this substance is likely to occur from:
outdoor use,
indoor use (e.g. machine wash liquids/detergents, automotive care products, paints and coating or adhesives, fragrances and air fresheners),
indoor use in close systems with minimal release (e.g. cooling liquids in refrigerators, oil-based electric heaters) and outdoor use in close systems with minimal release (e.g. hydraulic liquids in automotive suspension, lubricants in motor oil and break fluids). |




![cyclopenta[def]phenanthrene](/upload/2023/8/6ddc9c4b-5bd0-48be-9ccf-ec5942d01e33.png)
![4H-Cyclopenta[def]phenanthrene](/upload/2023/8/7b8f8597-3c30-4fe6-ad7a-7e903dbf7ca3.png)
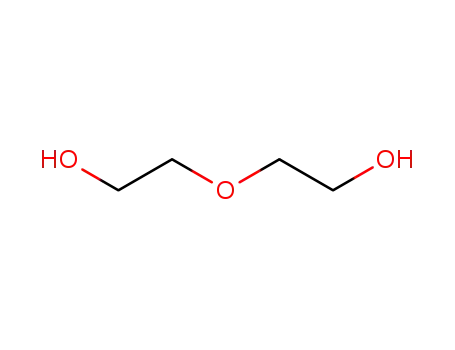
![2,2'-[1,2-ethanediylbis(oxy)]bisethanol](/upload/2023/8/42b15671-9359-41bc-a830-494a11705efd.png)