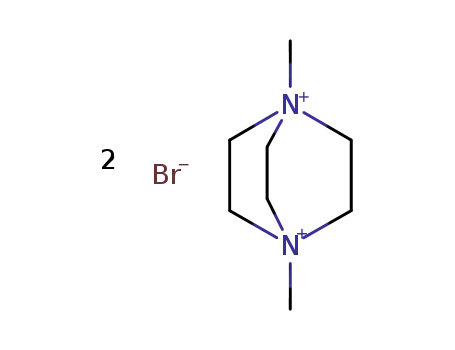Buy Reliable Quality High Purity 99% Triethylenediamine 280-57-9 Efficient Delivery
- Molecular Formula:C6H12N2
- Molecular Weight:112.175
- Appearance/Colour:white crystalline powder
- Vapor Pressure:2.9 mm Hg ( 50 °C)
- Melting Point:156-159 °C(lit.)
- Refractive Index:n20/D 1.4634(lit.)
- Boiling Point:174 °C at 760 mmHg
- PKA:3.0, 8.7(at 25℃)
- Flash Point:62.2 °C
- PSA:6.48000
- Density:1.08 g/cm3
- LogP:-0.50660
Triethylenediamine(Cas 280-57-9) Usage
|
Chemical Properties
|
Triethylenediamine also known as DABCO or TEDA, is a highly symmetrical molecule with a cage structure. The colorless extremely hygroscopic crystals is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent in polymerization and organic synthesis. |
|
Uses
|
An anti-fade reagent shown to scavenge free-radicals due to flurochrome excitation. |
|
Definition
|
ChEBI: Triethylenediamine is an organic heterobicylic compound that is piperazine with an ethane-1,2-diyl group forming a bridge between N1 and N4. It is typically used as a catalyst in polymerization reactions. It has a role as a catalyst, a reagent and an antioxidant. It is a bridged compound, a tertiary amino compound, a saturated organic heterobicyclic parent and a diamine. |
|
Preparation
|
Triethylenediamine can be produced from ethylenediamine or ethanolamine, diethanolamine, or diethylenetriamine with a variety of different catalysts. |
|
Reactions
|
Triethylenediamine reacts virtually quantitatively with bromine to give a 1/1 adduct. With alkyl halides it forms quaternary salts, even in nonpolar solvents. Apart from its highly nucleophilic nature, triethylenediamine exhibits catalytic activity in base-catalyzed reactions. |
|
General Description
|
Dabco?33-LV (Db) is a gelling catalyst and a bidentate ligand that forms a self-assembled monolayer (SAM) on a variety of substrates. It functionalizes the surface and immobilizes the surface atoms. |
|
Hazard
|
Skin irritant. |
|
Flammability and Explosibility
|
Flammable |
|
Purification Methods
|
DABCO crystallises from 95% EtOH, pet ether or MeOH/diethyl ether (1:1). Dry it under vacuum over CaCl2 and BaO. It can be sublimed in vacuo, and readily at room temperature. It has also been purified by removal of water during azeotropic distillation of a *benzene solution. It is then recrystallised twice from anhydrous diethyl ether under argon, and stored under argon [Blackstock et al. J Org Chem 52 1451 1987]. [Beilstein 23/3 V 487.] |
InChI:InChI=1/C6H12N2/c1-2-8-5-3-7(1)4-6-8/h1-6H2
280-57-9 Relevant articles
-
Shishkin,G.V.,Anisimova,I.L.
, (1978)
-
-
Anderson et al.
, (1967)
-
CHARACTERIZATION OF TRANSIENT INTERMEDIATES ON LASER FLASH EXCITATION OF CYCLOHEXENONES IN THE PRESENCE OF AMINES
Dunn, D. A.,Schuster, D. I.,Bonneau, R.
, p. 2802 - 2804 (1985)
-
Generation of a Mn(IV)-Peroxo or Mn(III)-Oxo-Mn(III) Species upon Oxygenation of Mono- and Binuclear Thiolate-Ligated Mn(II) Complexes
Lee, Chien-Ming,Wu, Wun-Yan,Chiang, Ming-Hsi,Bohle, D. Scott,Lee, Gene-Hsiang
, p. 10559 - 10569 (2017)
A thiolate-bridged binuclear complex [PP...
Degradation of Organic Cations under Alkaline Conditions
You, Wei,Hugar, Kristina M.,Selhorst, Ryan C.,Treichel, Megan,Peltier, Cheyenne R.,Noonan, Kevin J. T.,Coates, Geoffrey W.
supporting information, p. 254 - 263 (2020/12/23)
Understanding the degradation mechanisms...
Synthesis method of triethylene diamine
-
Paragraph 0031; 0033-0036; 0038-0041; 0043-0046; 0048-0051, (2020/09/16)
The invention relates to a synthesis met...
METHOD FOR PRODUCING PIPERAZINE AND TRIETHYLENEDIAMINE
-
Paragraph 0055; 0056; 0057; 0058, (2017/07/19)
PROBLEM TO BE SOLVED: To provide a metho...
METHOD FOR PRODUCING BICYCLIC AMINE COMPOUND
-
Paragraph 0047; 0054, (2017/11/15)
PROBLEM TO BE SOLVED: To provide a stabl...
280-57-9 Process route
-
-
73997-41-8
1-Phenethyl-4-aza-1-azonia-bicyclo[2.2.2]octane
-
-
280-57-9,88935-43-7
1,4-diaza-bicyclo[2.2.2]octane
-
-
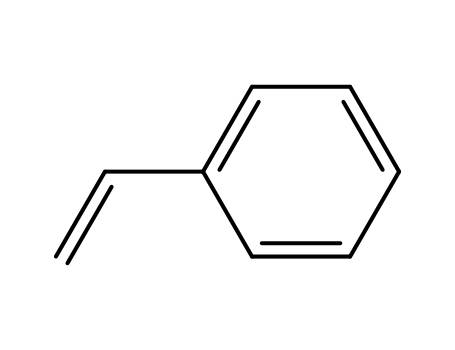
100-42-5,25038-60-2,25247-68-1,28213-80-1,28325-75-9,79637-11-9,9003-53-6
styrene
Conditions
| Conditions |
Yield |
|
With
potassium hydroxide; potassium chloride;
In
water;
at 40 ℃;
Mechanism;
Rate constant;
further base-solvent system;
|
|
-
-
280-57-9,88935-43-7
1,4-diaza-bicyclo[2.2.2]octane
Conditions
| Conditions |
Yield |
|
With
Rh/Al2O3; hydrogen;
In
tetrahydrofuran;
at 175 ℃;
under 37503.8 Torr;
Reagent/catalyst;
Temperature;
Solvent;
Sealed tube;
|
87.6%
|
280-57-9 Upstream products
-
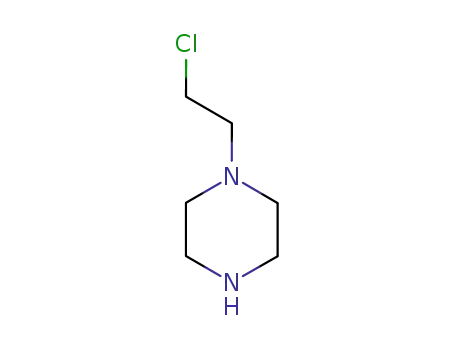
61308-25-6
Chloroethylpiperazine
-
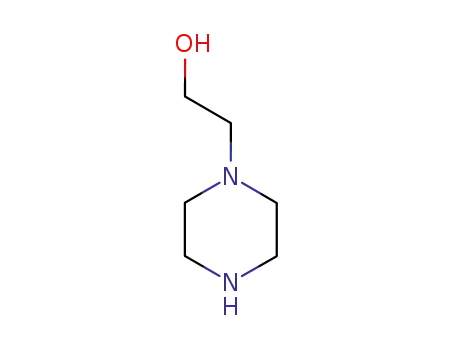
103-76-4
1-(2-hydroxyethyl)piperazine
-
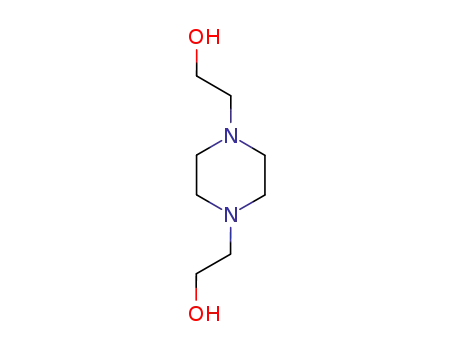
122-96-3
1,4-bis(2-hydroxyethyl)piperazine
-
14426-21-2
diethanolamine hydrochloride
280-57-9 Downstream products
-
5450-74-8
1,4-dimethyl-1,4-diazabicyclo[2.2.2]octane-1,4-diium dibromide
-
42790-43-2
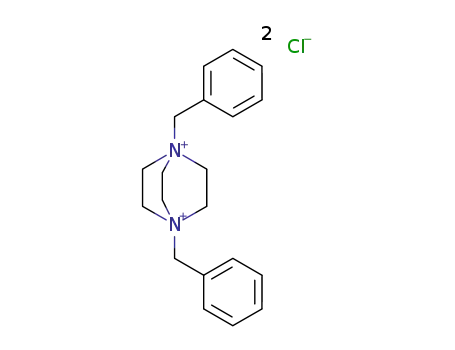
N,N'-dibenzyl-1,4-diazonia bicyclo[2.2.2]octane dichloride
-
14870-72-5
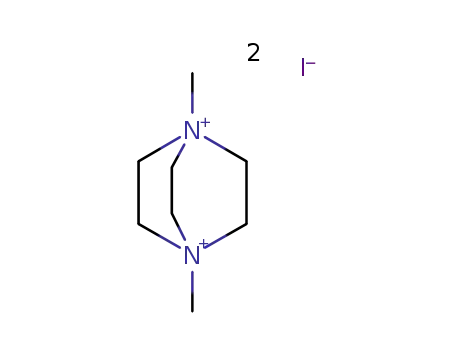
1,4-dimethyl-1,4-diazabicyclo[2.2.2]octane-1,4-diium diiodide
-
93-97-0
benzoic acid anhydride


![1-Phenethyl-4-aza-1-azonia-bicyclo[2.2.2]octane](/upload/2023/8/0d7453ea-08ee-440a-ad75-f555717d2975.png)

![1,4-diaza-bicyclo[2.2.2]octane](/upload/2023/8/0e611909-06e6-4923-99a3-ad891ad08bb8.png)