|
Description
|
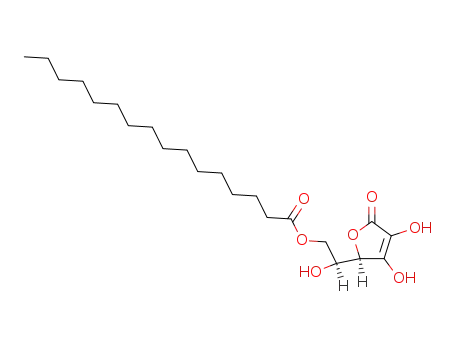
Ascorbyl palmitate is a lipophilic derivative of ascorbic acid with antioxidant and antiproliferative activities. It scavenges hydroxyl radicals in cell-free assays. Ascorbyl palmitate (0.01%) reduces the rate of autoxidation of soybean, safflower, sunflower, peanut, and corn oil. It inhibits increases in epidermal ornithine decarboxylase activity and DNA synthesis induced by phorbol 12-myristate 13-acetate (TPA; ) in mice in a concentration-dependent manner when applied topically. Ascorbyl palmitate (0.8 and 4 μmol per 200 μl of acetone, applied topically) reduces the number of tumors per mouse and the percentage of mice with tumors in a mouse skin two-stage model of tumor formation initiated and promoted by 7,12-dimethylbenz[a]anthracene (DMBA) and TPA, respectively. Formulations containing ascorbyl palmitate have been used as antioxidants and preservatives in foods, pharmaceuticals, and cosmetics. |
|
Chemical Properties
|
Ascorbyl palmitate is a white or yellowish-white solid with a soapy taste and a citrus-like odor. It is very slightly soluble in water but freely soluble in alcohol, animal oil, and vegetable oil. It melts at 107° to 117°C (around 234°F). Ascorbyl palmitate prevents oxidative rancidity development by quenching singlet oxygen. The ascorbic acid part of the molecule donates hydrogen (a reducing agent). This phenomenon is also called "oxygen scavenging". |
|
Uses
|
Ascorbyl Palmitate is an ester formed from ascorbic acid and palmitic acid creating a fat soluble form of vitamin C. It is used as an antioxidant food additive. It is also used as a preservative and an anti-oxidant in cosmetic creams and lotions to prevent rancidity. Ascorbyl palmitate facilitates the incorporation of ingredients such as vitamins A, C, and D into cosmetic formulations. It has no known toxicity. |
|
Definition
|
ChEBI: Ascorbyl palmitate is a fatty acid ester. It is derived from corn dextrose fermentation and palm oil. It is used as a food preservative and as an antioxidant in oils, fats, and pharmaceuticals. |
|
Production Methods
|
Ascorbyl palmitate is prepared synthetically by the reaction of
ascorbic acid with sulfuric acid followed by reesterification with
palmitic acid. |
|
Application
|
Ascorbyl Palmitate is an antioxidant formed by combining ascorbic acid with palmitic acid. ascorbic acid is not fat soluble but ascorbyl palmitate is, thus combining them produces a fat-soluble antioxidant. it exists as a white or yellowish white powder of citric- like odor. it is used as a preservative for natural oils, edible oils, colors, and other substances. it acts synergistically with alpha-tocopherol in oils/fats. it is used in peanut oil at a maximum level of 200 mg/kg individually or in combination. |
|
General Description
|
A white or yellowish-white powder having a citrus-like odor. Mp 116–117°C; soluble in alcohol and in animal and vegetable oils; slightly soluble in water. Ascorbyl Palmitate is a lipophilic ascorbic acid derivative, used as an antioxidant in both food and cosmetics industries. |
|
Pharmaceutical Applications
|
Ascorbyl palmitate is primarily used either alone or in combination
with alpha tocopherol as a stabilizer for oils in oral pharmaceutical
formulations and food products; generally 0.05% w/v is used. It
may also be used in oral and topical preparations as an antioxidant
for drugs unstable to oxygen. The combination of ascorbyl
palmitate with alpha tocopherol shows marked synergism, which
increases the effect of the components and allows the amount used
to be reduced.
The solubility of ascorbyl palmitate in alcohol permits it to be
used in nonaqueous and aqueous systems and emulsions. |
|
Biochem/physiol Actions
|
6-O-Palmitoyl-L-ascorbic acid possesses metastasis inhibitory and anti-tumor activity. Ascorbyl palmitate exhibits antioxidant activity by protecting the cell membranes from oxidative damage. |
|
Safety Profile
|
When heated to
decomposition it emits acrid smoke and
irritating fumes. |
|
Safety
|
Ascorbyl palmitate is used in oral pharmaceutical formulations and
food products, and is generally regarded as an essentially nontoxic
and nonirritant material. The WHO has set an estimated acceptable
daily intake for ascorbyl palmitate at up to 1.25 mg/kg bodyweight.
LD50 (mouse, oral): 25 g/kg
LD50 (rat, oral): 10 g/kg |
|
storage
|
Ascorbyl palmitate is stable in the dry state, but is gradually
oxidized and becomes discolored when exposed to light and high
humidity. In an unopened container, stored in a cool place, it has a
shelf life of at least 12 months. During processing, temperatures
greater than 658℃ should be avoided.
The bulk material should be stored in an airtight container at
8–158℃, protected from light. |
|
Incompatibilities
|
Incompatibilities are known with oxidizing agents; e.g. in solution
oxidation is catalyzed by trace metal ions such as Cu2+ and Fe3+. |
|
Regulatory Status
|
GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (oral, rectal,
topical preparations). Included in nonparenteral medicines licensed
in the UK. |
|
Who Evaluation
|
Evaluation year: 1973 |